State of the fertility sector 2022/23
Annual publication on HFEA licensed centres
(1st April 2022 – 31st March 2023)
Published: 12th September 2023
Download the underlying dataset as .xlsx
Table of contents
- About this report
- 1. There were 85 inspections undertaken in 2022/23
- 2. Critical non-compliances have decreased to 2%
- 3. In 2022/23, 107 clinics were licensed by the HFEA to provide fertility treatment
- 4. No Grade A incidents since 2020/21, and severe/critical OHSS incidents remained consistent with previous years
- 5. Patient complaints decreased in 2022/23
- About our data
About this report
The Human Fertilisation and Embryology Authority (HFEA) is the independent regulator of fertility treatment and human embryo research in the United Kingdom (UK). We aim to ensure that everyone receives high quality care in a licenced fertility clinic. We do this by licensing, monitoring, and inspecting fertility clinics and research centresi, and providing free, clear and impartial information about fertility treatment, clinics, and egg, sperm, and embryo donation.
The ‘State of the fertility sector’ summarises what we have seen through our regulatory work during the year. The report is published annually and is compiled from information gathered from our inspections and from other sources of information including our Register of fertility treatments, incident reports, and patient feedback mechanisms.
We have continued to inspect the centres we license using the hybrid inspection methodology introduced during the COVID-19 pandemic. You can find out more about the HFEA regulatory response to COVID-19 and the subsequent changes made to inspections in the State of the fertility sector 2020/21 report, and the impact on treatment in the Impact of COVID-19 on fertility treatment 2020 report. Some of the data in this year’s State of the fertility sector report was impacted by the pandemic, which means data from 2020/21 and 2021/22 may not be directly comparable with other years.
In 2019/20 we introduced Quarterly Clinical Governance Updates (via our Clinic Focus newsletter) which provide centres with detailed insight into non-compliances in a timely manner to help them improve compliance and prepare for inspections. These updates also contain details of incidents and complaints to ensure centres can implement any recommendations to help prevent further recurrence. As a result, this State of the fertility sector report provides a high-level overview of sector-wide compliance, reporting on the absolute number of inspections, non-compliances, incidents, and complaints.
1. There were 85 inspections undertaken in 2022/23
Since 2020/21, centres have been assessed using a hybrid approach involving a desk-based assessment combined with an onsite visit to allow continued close regulatory oversight of the fertility sector. This approach is efficient, allowing the inspector to focus on specific areas of concern whilst reducing the time spent onsite. More information on changes to inspections can be found in the State of the fertility sector 2020/21 report.
85 inspections were carried out in 2022/23 which is a decrease from 2021/22 (Figure 1). This is primarily due an increased number of inspections being conducted in 2021/22 as inspections were deferred from 2020/21 during the COVID-19 pandemic.
Following each clinic or research centre inspection, a report identifying areas of good practice and those which are non-compliant and require improvement is published on the HFEA website via the respective centre Choose a Fertility Clinic webpage. In 2022/23, 46 (54%) inspections were renewalsii, and 23 (27%) were interimii. One interim inspection and six renewal inspections were cancelled, due to voluntary revocations of license, which are not included in this data. Additionalii inspections were conducted where significant concerns had been identified.
Figure 1. There were 85 inspections undertaken in 2022/23
Number of inspections by type, 2018/19-2022/23
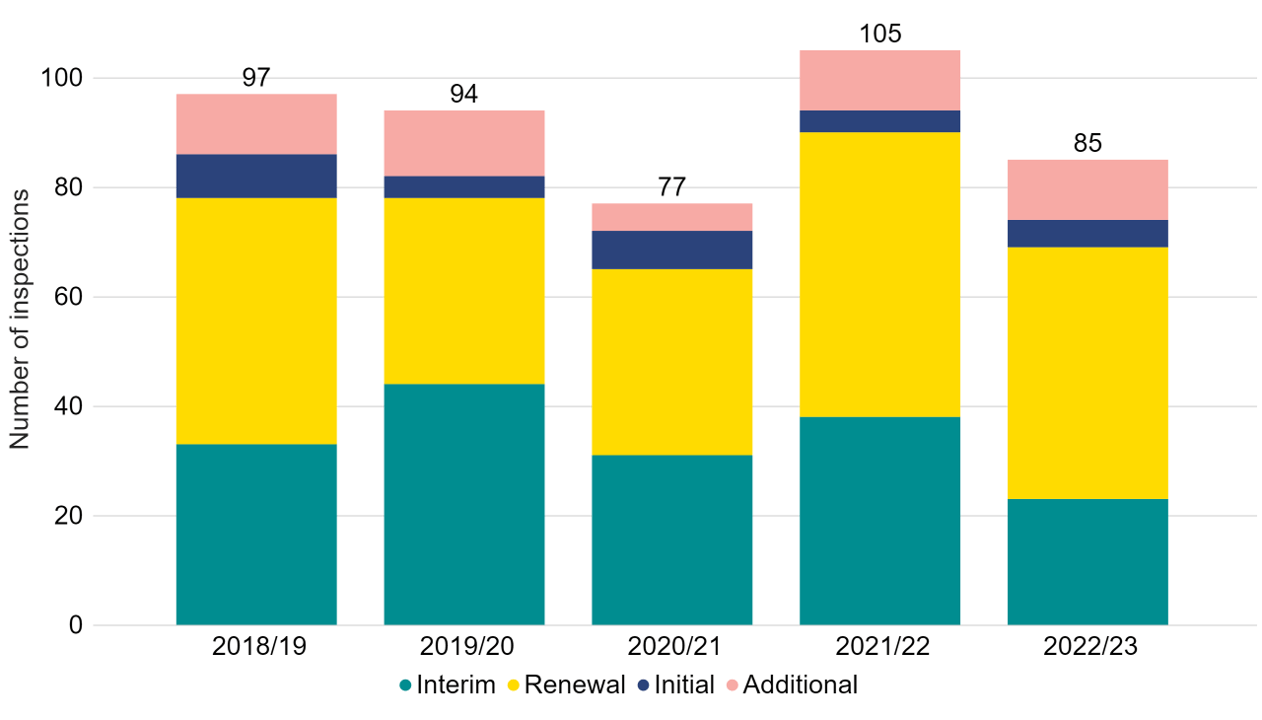
Note figure 1: Some inspections in 2020/21 were deferred until 2021/22 due to the COVID-19 pandemic. This data does not include 1 interim inspection, and 6 renewal inspections, which were cancelled. 3 focussed inspections were assigned to Interim inspections in this data. 1 change of premises inspection and 1 additional targeted interim inspection were assigned to additional inspections in this data.
Download the underlying data for Figure 1 as Excel Worksheet
2. Critical non-compliances have decreased to 2%
Following each clinic or research centre inspection, a report identifying areas of good practice and those which are non-compliant and require improvement is published. The HFEA may issue actions, if necessary, to address any areas of non-compliance or partial compliance. Each area of non-compliance is identified in reference to the relevant sections of the Human Fertilisation and Embryology (HFE) Act 1990 (as amended), Standard licence conditions, General directions, or the Code of Practice. These non-compliances are classified as ‘critical’, ‘major’ or ‘other’iii. Centres are required to implement these actions within specified timescales and progress is monitored by the HFEA Inspection team. Further information about how the HFEA handles non-compliance can be found in the HFEA Compliance and Enforcement policy.
In 2022/23, 187 non-compliances were identified during inspections (Figure 2). Of these, 96 were identified as ‘major’ and 87 ‘other’ non-compliances. Only 2% of non-compliances (five) were assessed as ‘critical’ which has decreased from 4% in 2021/22. You can find out more about the types of non-compliances identified in 2022/23 in our Quarterly Clinical Governance Updates.
Figure 2. Only 2% of non-compliances were graded critical in 2022/23
Percentage of non-compliances by grade, 2022/23
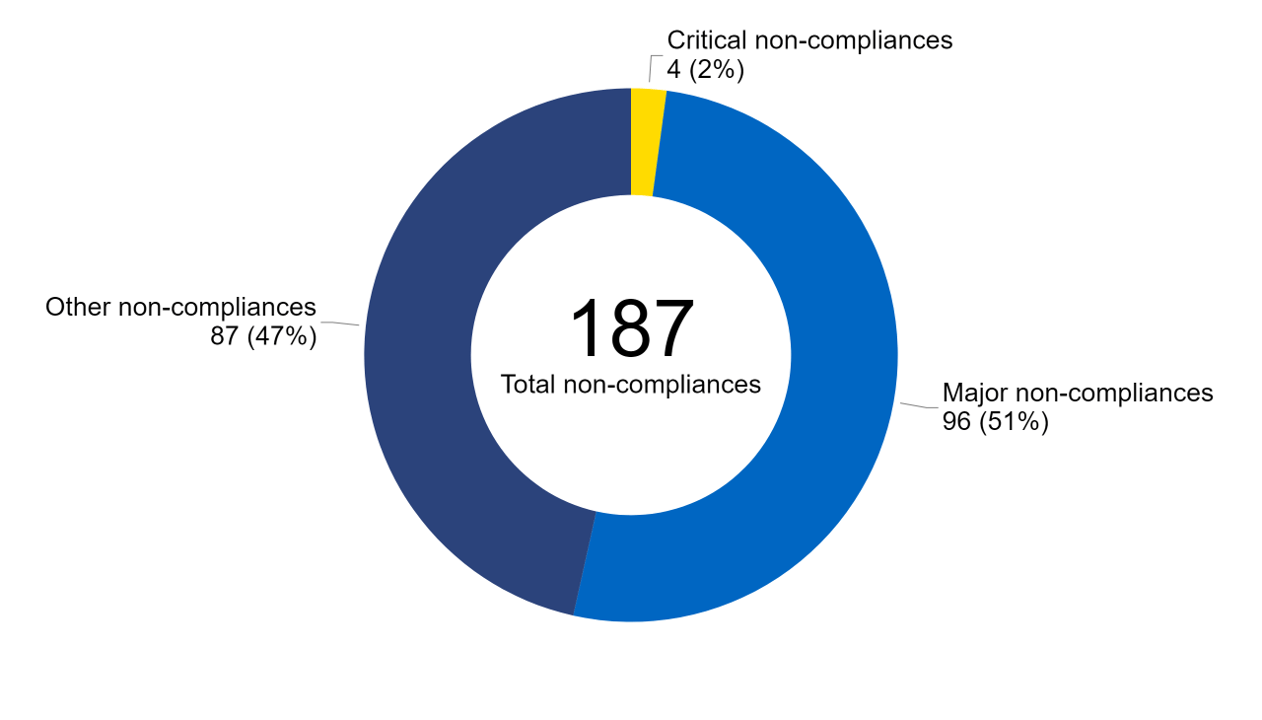
Note figure 2: Non-compliance data from inspection reports relating to six clinics have yet to be finalised at the date of data collection.
Download the underlying data for Figure 2 as Excel Worksheet
3. In 2022/23, 107 clinics were licensed by the HFEA to provide fertility treatment
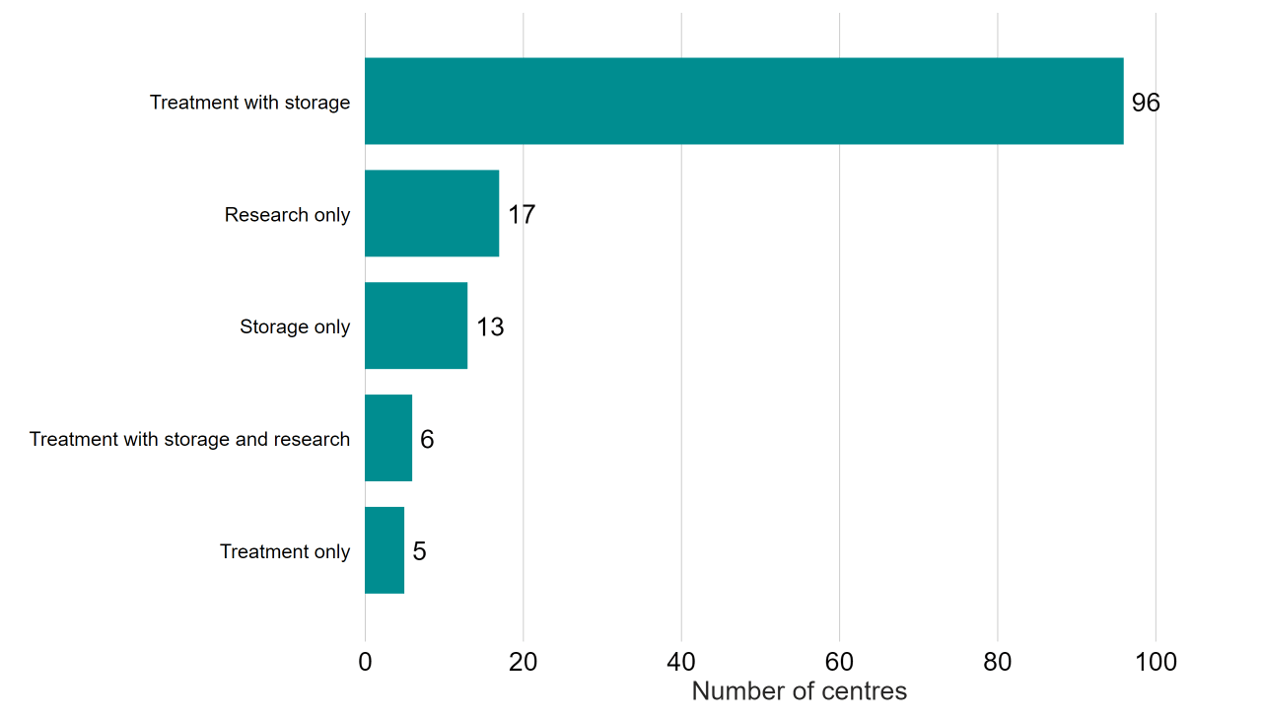
In 2022/23 there were 107 fertility clinics licensed by the HFEA to provide fertility treatment in the UK (Figure 3). An additional 17 establishments were licensed to undertake research and 13 were licensed to provide storage only.
Of the 107 licensed treatment clinics, 66 (62%) were privately owned, an increase from 62 in 2021/22. It should be noted that most HFEA licensed clinics, whether private or NHS, treat both NHS and self-funded patients.
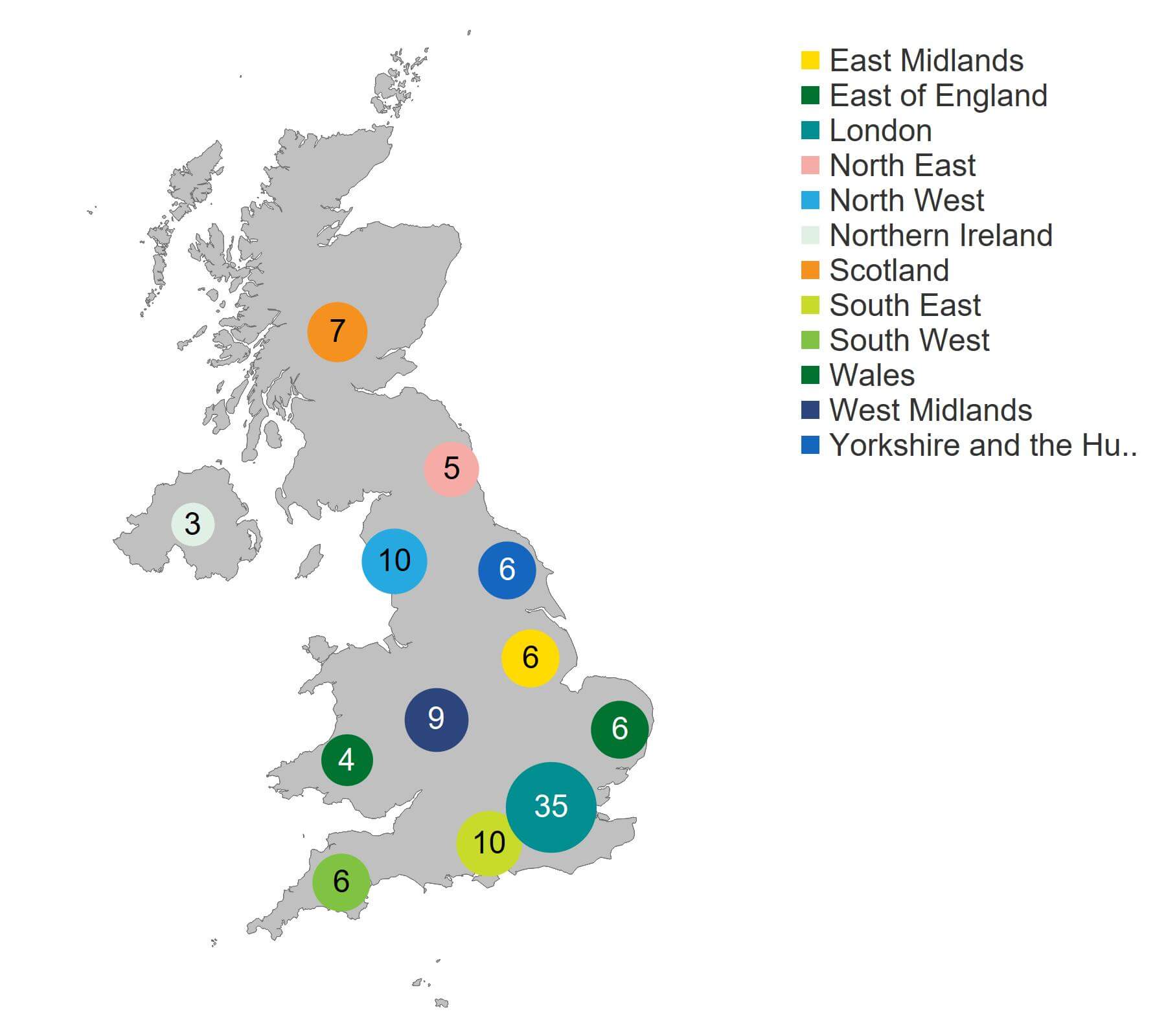
The largest number of clinics offering fertility treatment was based in London (35 clinics; Figure 4), followed by the North West and South East (10 clinics each) and West Midlands (9 clinics). Northern Ireland had the fewest treatment clinics at three.
Figure 3. Most HFEA licensed centres provided both treatment and storage to their patients
Number of licensed centres by type, 2022/23
Download the underlying data for Figure 3 as Excel Worksheet
Figure 4. London had the highest number of HFEA licensed fertility clinics at 35
Number of clinics licensed to provide fertility treatment by geographical area, 2022/23 (excluding storage and research only)
Download the underlying data for Figure 4 as Excel Worksheet
4. No Grade A incidents since 2020/21, and severe/critical OHSS incidents remained consistent with previous years
Around 100,000 treatment and storage cycles are carried out each yeariv, in addition to around 1,200 new egg donor registrationsv, and over 99% of these are carried out without any incidents being reported. Of those incidents, very few are of the most serious type – Grade Avi. Centres and the HFEA take adverse incidents very seriously, and we have a rigorous process for reporting, handling, and investigating adverse incidents and near misses (see the Code of Practice and Standard licence conditions for more information). Reporting adverse incidents in In vitro Fertilisation (IVF) is a statutory requirement but it is also recognised as one of the best ways of ensuring that incidents and their causes are identified. This process allows centres across the sector to learn from incidents and ensure changes can be made to prevent reoccurrence. We monitor incidents in centres to make sure that everything is done to understand what went wrong and, crucially, to take steps to ensure it does not happen again. We also share learning and notify other centres of potential issues to ensure the sector as a whole learns from this. The Quarterly Clinical Governance Updates provide centres with detailed insight into incidents.
There were 517 incidents and 89 near misses reported to the HFEA in 2022/23 (Figure 5). Just over half of all incidents reported were Grade C (291 reported incidents) with the remainder being Grade B (226 reported incidents). There were no Grade A incidents from 2020/21 to 2022/23.
Field Safety Notices (FSNs) are important communications about the safety of a medical device. These are sent to the Medicines and Healthcare products Regulatory Agency (MHRA) in the first instance, who notify us in turn. FSNs contain information about what centres need to do to reduce the specified risks of using the affected medical device(s) and set out any corrective action that they need to undertake. Our role is to communicate each FSN to the HFEA licensed centres so they can implement the required steps. There were 10 FSNs issued by the HFEA in 2022/23 relating to medical devices used in licensed centres.
Figure 5. 517 incidents reported in 2022/23
Number of incidents reported by severity, 2022/23
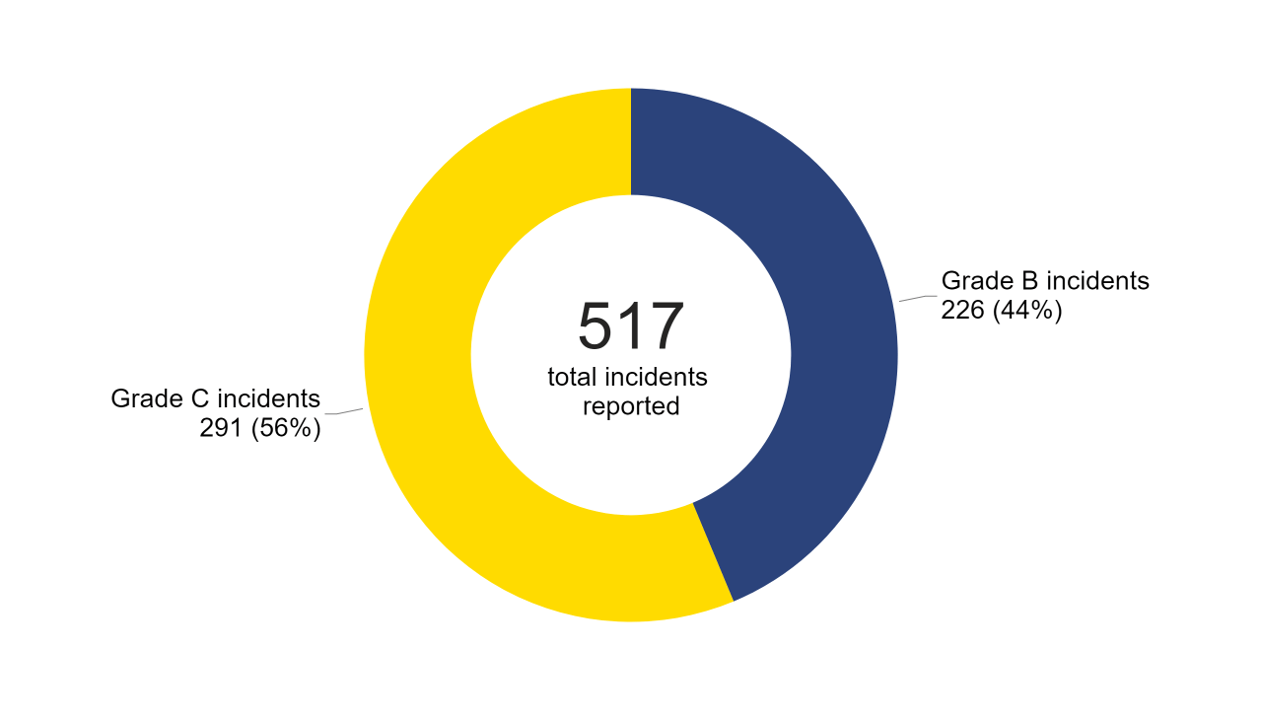
Note figure 5: This data includes 517 incidents, including 64 incidents which relate to severe and/or critical OHSS.
Download the underlying data for Figure 5 as Excel Worksheet
Total reported incidents decreased from 671 incidents in 2021/22 to 517 in 2022/23. Near misses also decreased between 2021/22 and 2022/23, from 122 near misses to 89 respectively. This decrease is partially due to changes in how patient hospital admissions are reported by clinics and handled by the HFEA, particularly in relation to mild and moderate Ovarian Hyperstimulation Syndrome (OHSS).
OHSS is a potentially serious side effect of fertility drugs taken to increase egg production. OHSS can range in severity from mild to critical, and is graded by the patient’s clinic in accordance with guidelines by the Royal College of Obstetricians and Gynaecologists. Because of the potentially serious nature of OHSS, all cases of severe or critical OHSS must be reported to the HFEA within 24 working hours by the patient’s clinic and are generally categorised as Grade B incidents.
During the COVID-19 pandemic, HFEA guidance temporarily required clinics to report all hospital admissions, including mild/moderate OHSS, to help reduce the burden on the NHS at a time of increased stress. These mild and moderate OHSS incidents were temporarily categorised as Grade C incidents. HFEA guidance was updated in February 2022 and clinics were no longer required to report all patient hospital admissions. This has led to a decrease in Grade C incidents in 2022/23. Some clinics have continued to take a cautious approach and report mild and moderate OHSS incidents, even though it is no longer necessary under current requirements. Because of the continued incident reports which relate to mild or moderate OHSS, from September 2022 these reports are now being categorised as ‘not an incident’ in line with our current practice.
We have worked with clinics to ensure they do everything possible to prevent and manage OHSS. In 2022/23, there were 64 cases of severe and critical OHSS reported by UK clinics, occurring in less than 0.1% of cycles (Figure 6).
Figure 6. Severe and critical OHSS incidents remain consistent with previous years
Number of severe and critical OHSS incidents reported 2018/19-2022/23
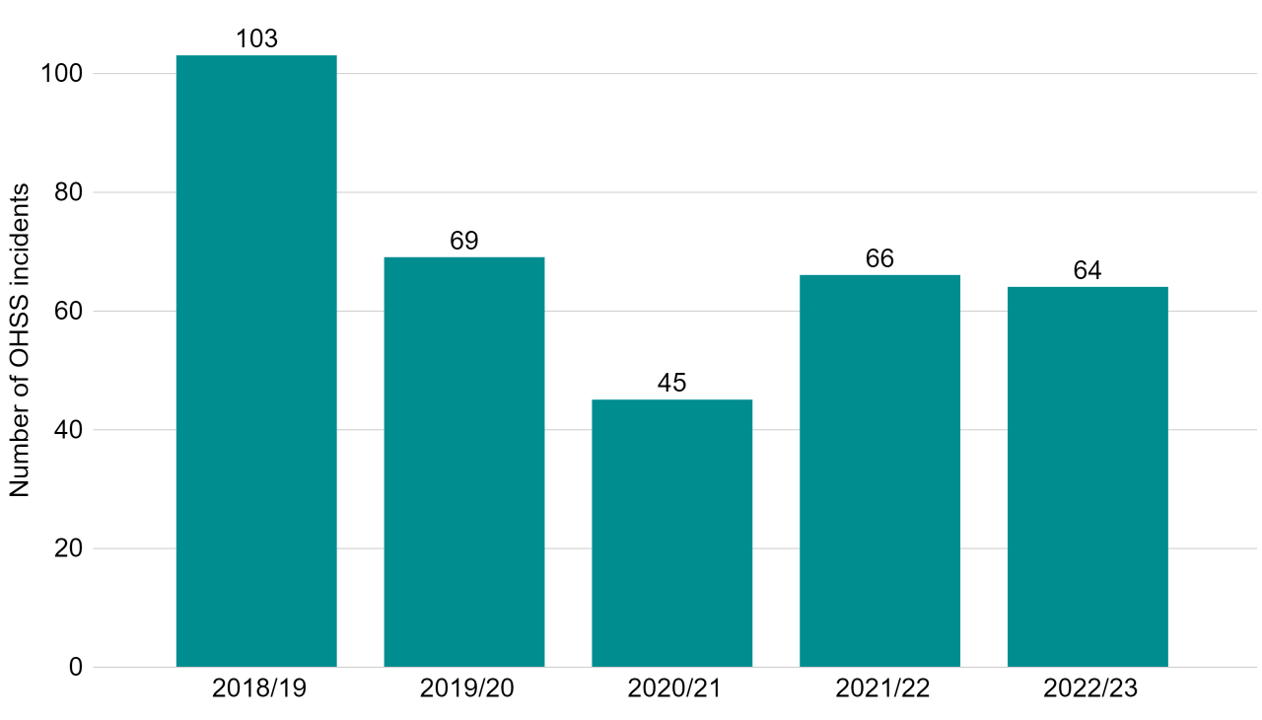
Note figure 6: This data includes incidents which related to severe and/or critical OHSS. These severe and critical OHSS incidents are also included in the incident totals provided in Fig 5. Mild and moderate OHSS cases are not included in this data.
Download the underlying data for Figure 6 as Excel Worksheet
5. Patient complaints decreased in 2022/23
Patients and donors can raise a complaint with us if they are unhappy with how a clinic has handled their original complaint. However, unlike some other regulatory bodies, the HFEA does not have a statutory duty to investigate patient complaints. We can only consider a complaint made by a patient or donor where the complaint indicates a potential breach of the HFE Act 1990 (as amended), licence conditions, or the guidance set out in the Code of Practice. This means we may not be able to review specific medical decisions made during a patient’s care pathway, nor can we intervene to require a clinic to provide a refund or financial compensation. The number of patient complaints handled by the HFEA is therefore not a reliable indicator of the levels of patient satisfaction.
Around 60,000 patients undergo treatment, storage, and/or donation cycles per yeariv-v. About 0.1% of these patients submit complaints to the HFEA. Complaints received are often multifaceted and the complexity of patient complaints continues to increase. We publish the Quarterly Clinical Governance Updates to provide clinics with detailed insight into complaints.
In 2022/23, many of the complaints we received related to decisions made during a patient’s care pathway and poor communication. We also received several patient complaints about the lack of engagement once a formal complaint was made with their clinic. We strongly urge clinics to review their complaint policies to ensure complaints are acknowledged and responded to within their policy timeframes. If a complaint response is going to be delayed, we urge clinics to explain the reason for the delay and offer an apology as well as providing a revised timeframe for the response.
We actively monitor and review the information we receive from patient complaints and where there has been a potential breach of HFE Act 1990 (as amended), Code of Practice, or license conditions, we feed this into the inspection process. We discuss formal complaints with the clinic and encourage them to further engage with the complainant to try to locally resolve the issues raised. Complaints are discussed at regular HFEA internal clinical governance meetings to ensure clinics are actively engaging with patient complaints and providing thorough and well considered responses. We expect clinics to provide a sincere acknowledgement of the complainant’s experience; to explain what, if anything, went wrong; what measures the clinic has put in place to minimise the risk of this happening again and what the clinic can offer the complainant by way of support.
There were 59 patient complaints received in 2022/23, which has decreased since the previous year (Figure 7). This includes 12 formal complaints and 47 informal complaintsvii.
As well as our complaint handling service we also receive a large volume of patient queries via our general enquiries on how to raise a complaint about a clinic. You can find out more about making a complaint on our Problems at your fertility clinic webpage.
Figure 7. The number of complaints received has decreased since 2020/21
Number of informal and formal complaints received, 2018/19-2022/23
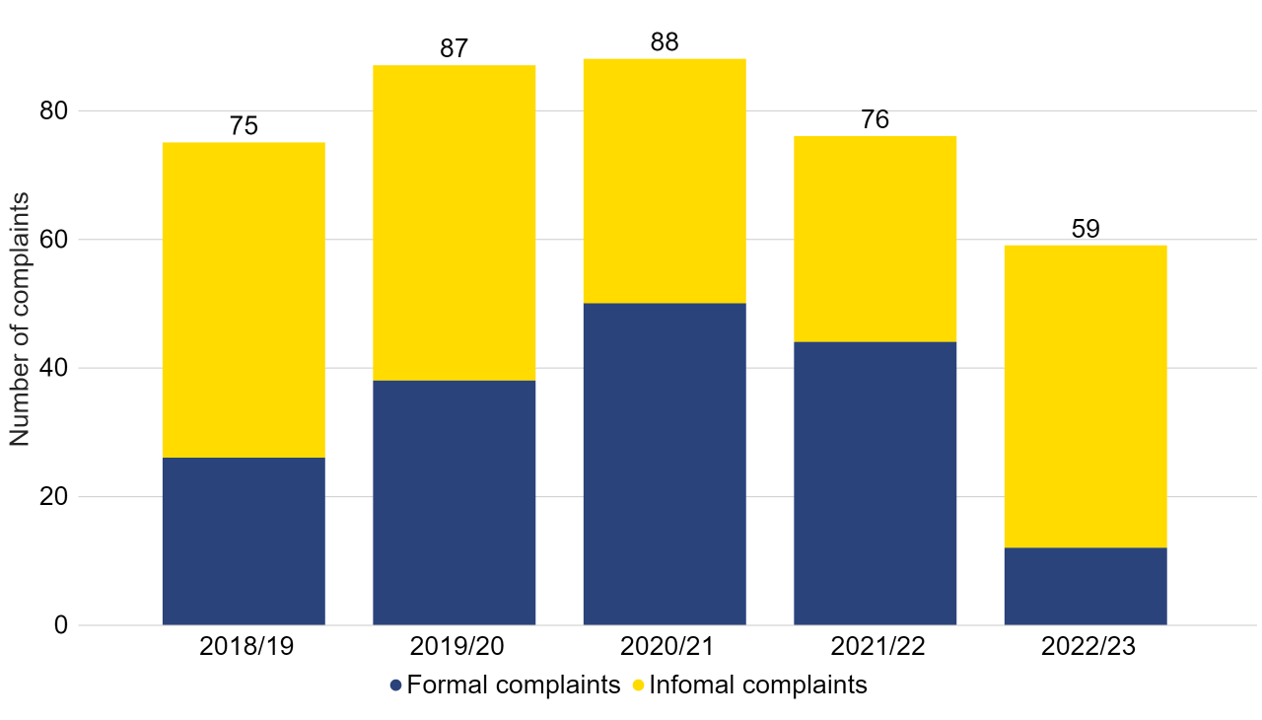
Download the underlying data for Figure 7 as Excel Worksheet
About our data
This report is compiled from information gathered from our inspections throughout the year and other sources of information including incident reports, patient feedback, and patient complaints.
This report also uses preliminary treatment data for 2021 from the HFEA Register of fertility treatments. The HFEA has recently launched a new data submission system for licensed clinics and have migrated our fertility treatment and outcomes data to a new database. This data migration has resulted in delays which has prevented the validation of the 2020-2021 treatment and pregnancy data and 2019-2021 birth outcome data. Data validation involves data quality checks that verify treatment, pregnancy and birth outcome data.
The information that we publish is a snapshot of data provided to us by licensed clinics. The figures supplied in this report are from our data warehouse containing Register data as of 19 April 2023. Results are published according to the year in which the cycle was started.
A full list of definitions of classification of incidents and complaints are available in the underlying dataset.
An update to the underlying dataset has been provided to correct data on total OHSS incident report counts for 2022/23. The data previously overreported mild/moderate OHSS cases included in the total by five cases.
Contact us regarding this publication
Media: press.office@hfea.gov.uk
Statistical: intelligenceteam@hfea.gov.uk
Accessibility: comms@hfea.gov.uk
For general information about the HFEA, fertility treatments, and research activity please visit the HFEA website. For guidance on how to make a complaint about a clinic, please visit our Problems at your fertility clinic webpage.
Notes on State of the Sector 2021/22
- Establishments licensed by the HFEA are defined as:
- Centre: a broad category which includes any HFEA licensed establishment; this includes those that offer treatment, storage, or research and/or a combination of the above.
- Clinic: relates specifically to a centre which is licensed to offer treatment to fertility patients, either as a treatment only clinic, or as a treatment clinic which may also offer storage and/or carry out research.
- Inspections are carried out by the HFEA to assess whether centres comply with essential requirements in providing safe and high-quality care to patients and donors. Inspections are categorised as follows:
- Initial: The first inspection in relation to a new clinic license application, which covers all areas of regulatory compliance. New licensed centres usually receive a licence to operate for up to two years for an initial licence, however established centres and new centres which are part of a fertility clinic group generally receive a licence for up to 4 years. (five years is the maximum length of a treatment licence permitted by law).
- Interim: An inspection which assesses set themes, as well as any non-compliances identified in previous inspections. Interim inspections include a shorter site visit, are unannounced and are usually carried out at the midpoint of the existing license.
- Renewal: An inspection which includes a thorough assessment of all aspects of regulatory compliance, as well as any non-compliances identified in previous inspections. As clinics are usually granted a 4 year licence the renewal inspection is carried out every four years.
- Additional: These inspections are usually focussed on a particular area of regulatory compliance, and may be carried out in a number of circumstances including by request of the HFEA license committee, in the event of a variation of premises, in response to a serious incident and/or whistleblowing, or in the event of any other concerns identified.
- Non-compliances are graded as:
- Critical: an area of practice which poses a significant risk of causing harm to a patient, donor, embryo or to a child who may be born as a result of treatment services, or a significant shortcoming from the statutory requirements.
- Major: an area of practice which poses an indirect risk to the safety of a patient, donor, embryo or to a child born as a result of treatment services. This area of non-compliance may also indicate a major shortcoming from the statutory requirements and/or indicate a failure by the Person Responsible to carry out their legal duties.
- Other: an ‘other’ area of practice that requires improvement is any area of practice, which cannot be classified as either a critical or major area of non-compliance, but which indicates a departure from statutory requirements or good practice.
- In 2021 there were approximately 100,000 cycles of treatment and storage involving approximately 60,000 patients:
- Approximately 85,200 treatment cycles (around 75,700 IVF; 7,100 DI; 2,400 genetic testing)
- Approximately 14,900 storage cycles (around 4,200 egg storage cycles; 10,700 embryo storage cycles)
- In 2020 there were approximately 1,200 new egg donor registrations
- Incidents are graded as:
- Grade A: involve severe harm to one person, or major harm to many
- Grade B: involves serious harm to one person, or moderate harm to many
- Grade C: involves minor harm
- Near miss: an event not causing harm, but has the potential to cause injury or ill health.
- Complaints are classified as:
- Formal: where the patient/donor has received a response from the clinic but remains dissatisfied, and/or where the clinic has entered into an extensive dialogue with the complainant and at the end of this process feel they have done all they can to resolve the complaint therefore advising the complainant to contact us. Complaints relating to an incident or complaints that relate to complex issues requiring further input from us are also classified as formal complaints.
- Informal: complaints that have not been raised with the clinic or complaints still going through the clinic’s complaint process.
Review date: 16 April 2026

